Last Revised: April 03, 2024
Last reviewed January 2022
Click here to return to Lab Safety Page Click here to jump to Section XVI: SOPs and Fact Sheets
The purpose of the Chemical Hygiene Plan is to acquaint students and employees with the University of Pennsylvania's safety and health policies and to inform employees of their rights and obligations under federal and state regulations. The University continually strives to provide a learning, teaching, and research environment free from recognized hazards. It is Penn's intention to improve the protection of the health and safety of students, employees and the public by providing access to information regarding the safe handling of chemicals and biological agents that are present in the workplace.
For Chemical Hygiene Plan questions or concerns, contact:
Karen Kelley, CIH, CHO
Associate Director, Industrial Hygiene and Lab Safety, Chemical Hygiene Officer
P: 267-667-2496
E: kelley@upenn.edu
Revised 3/2023
The CHP forms the foundation of the safe use of chemicals in the laboratory. It addresses chemical health and safety in Penn laboratories, and gives limited information about physical hazards commonly found in chemical research labs. The Chemical Hygiene Plan (CHP) must be available to laboratory workers, safety officers, and EHRS.
According to the US Occupational Safety & Health Administration (OSHA 29 CFR 1910.1450), federal regulations and standards, the Chemical Hygiene Plan (CHP) must include at a minimum:
- Standard operating procedures (SOPs) for each activity that uses hazardous materials. The SOPs may be generic in nature, that is, similar operations using chemicals of the same general class may be covered by one SOP.
- Criteria used to determine the risk associated with chemicals and the procedures used.
- Criteria used to determine and implement control measures to reduce laboratory workers' exposure to hazardous chemicals including engineering controls, the use of personal protective equipment, and hygiene practices. Particular attention must be given to the selection of control measures for chemicals known or suspected of being carcinogens, reproductive hazards, or acutely toxic chemicals.
- Provisions for laboratory worker training.
The Chemical Hygiene Plan applies to all laboratories at the University of Pennsylvania where hazardous chemicals are used, except those clinical laboratories located in the Hospital of the University of Pennsylvania (HUP). The hospital has its own Chemical Hygiene Plan.
The CHP is a requirement of the Occupational Safety and Health Administration regulation 29 CFR 1910.1450: “Occupational Exposure to Hazardous Chemicals in Laboratories”. This is commonly referred to as The Lab Standard.
Wherever hazardous chemicals are used in a research laboratory, a written CHP must be developed and implemented. The CHP must be capable of protecting laboratory workers and others from the health hazards associated with the hazardous chemicals used in the laboratory.
Not all chemical and physical hazard addressed by Penn’s CHP are present in every lab. The SOPs and Fact Sheets listed on your lab’s CHWP are the ones that apply to your lab’s hazards. You must read, understand, and comply with those SOPs and Fact Sheets that are listed on your lab’s CHWP.
The CHWP can be found in your lab’s profile in BioRAFT.
When a PI (or delegate) completes their lab's Lab Hazard survey in BioRAFT, a Safety Assurance & Chemical Hygiene Work Plan document is automatically generated. This document contains links to any Chemical Hygiene Plan SOPs and Fact Sheets that are applicable to the lab's hazards.
When the PI certifies the CHWP, it is automatically e-mailed to all lab members listed in BioRAFT. The lab members must then read the SOPs and Fact Sheets listed on the CHWP.
The Lab Hazards must be updated in BioRAFT at least annually or whenever there are changes to the hazards in the lab. The updated CHWP must then be resubmitted by the PI.
More information about completing the Lab Hazard survey and certifying the CHWP form can be found in Appendix B.
Deans and/or department chairpersons are responsible for establishing and maintaining compliance with the CHP. To this end, deans and department chairs may wish to designate safety officers within the schools or departments. A designated safety officer should hold the rank of associate professor or full professor.
The Principal Investigator is responsible for enforcing Penn's lab safety policies. This includes:
- Creating an expectation in his or her lab that proper lab attire and personal protective equipment (PPE) will be worn, and ensuring that PPE is maintained, replaced, and/or laundered when needed.
- Confirming that all lab members have completed any required EHRS lab safety training programs, as determined by Workday Learning.
- Maintaining an up to date Chemical Inventory in Penn's online chemical inventory system.
- Providing their lab members with a copy of the CHWP and creating an expectation that the lab members must read and comply with the SOPs and Fact Sheets listed on the CHWP.
- Timely reporting of unsafe conditions or events such as equipment malfunctions, near miss incidents, injuries, or facilities issues.
The Principal Investigator must ensure that their lab members have:
- Comprehensive, lab-specific safety training
- Opportunities for guidance and mentoring about safety issues, along with other research-related issues
- Personal protective equipment, including lab coats, eye protection and disposable gloves
- Regular opportunities to bring up safety concerns and questions without fear of retaliation
Individual laboratory workers are responsible for:
- Familiarizing themselves with the Standard Operating Procedures (SOPs) and Safety Fact Sheets that are listed on their lab’s CHWP.
- Planning and conducting each operation in accordance with the CHP Standard Operating Procedures (SOPs), Fact Sheets, and any applicable Hazard Control Plans written for their lab-specific processes.
- Wearing appropriate lab attire, a lab coat, and safety glasses when working in the laboratory.
- Developing good laboratory hygiene habits such as handwashing, housekeeping, maintaining PPE in good condition, etc.
- Reporting unsafe acts, injuries, spills, and near-misses to their Principal Investigator and Environmental Health and Radiation Safety.
The Executive Director of EHRS or his/her designee shall be the University Chemical Hygiene Officer.
The Office of Environmental Health and Radiation Safety (EHRS) is responsible for working with faculty, staff, students, and others to develop and implement appropriate chemical hygiene practices and procedures. To accomplish this:
EHRS establishes procedures to:
- Monitor the procurement, use, and disposal of chemicals used in laboratory.
- Conduct laboratory chemical hygiene inspections on a periodic basis and maintain records for those inspections.
- Assist PI's in the development of Hazard Control Plans for lab-specific hazards.
- Communicate the current legal requirements for regulated substances.
The Environmental Health and Radiation Safety office (EHRS) is responsible for recommending to the Vice Provost for Research the minimum requirements of the CHP that all laboratories must follow. EHRS will review the CHP at least annually.
Biological Safety
The safe use and storage of biological organisms requires control measures similar to those found in chemical safety. See the University of Pennsylvania Biological Safety Manual for proper work practices involving biological agents.
Radiation Safety
The safe use and storage of radioactive materials require control measures similar to those found in chemical safety. However, the use of radioactive materials has additional requirements. See the University of Pennsylvania Radiation Safety User’s Guide for safe work practices involving radioactive materials.
Revised 3/2023
The Chemical Hygiene Plan (CHP) provides the background information required to establish safe working practices for chemical use and handling. The responsibility for implementation and enforcement of safe work practices is the responsibility of the principal investigator of each laboratory. The Chemical Hygiene Plan functions as both a training tool and a reference.
The Office of Environmental Health and Radiation Safety has developed Standard Operating Procedures and Safety Fact Sheets for chemical and physical hazards commonly found in University Laboratories. These SOPs specify the minimum controls for safe use of hazardous materials and equipment at the University. Adherence to the SOPs by all University of Pennsylvania lab workers is mandatory. It is the responsibility of the Principal Investigator of each laboratory to review the SOPs and ensure that the protective equipment and procedures outlined are in place. The hazard-class SOPs included in this Chemical Hygiene Plan can be found in Section XVI along with instructions for creating task-specific Hazard Control Plans when needed.
The Principal Investigator must complete the laboratory hazard assessment questionnaire in BioRAFT each year; when research conditions change; or when new hazards are introduced to the lab environment. If the task of completing the laboratory hazard assessment questionnaire was delegated to a Lab Safety Coordinator, the PI must review the list and certify that the responses are accurate. Completion of the questionnaire will automatically assign relevant SOPs and fact sheets to the lab's chemical hygiene work plan form in BioRAFT.
Lab specific SOPs must be sent to EHRS to be uploaded to the lab’s documents section in BioRAFT.
The principal investigator must digitally sign the chemical hygiene work plan, which will then be automatically emailed to all the lab members listed in the lab member list in BioRAFT. The Chemical Hygiene Work Plan in BioRAFT supersedes the paper form of the Chemical Hygiene Work Plan.
Principal investigators must ensure that all laboratory workers are provided with information and training for the hazards present in their work area. Principal investigators are also responsible for ensuring that anyone conducting research activities in their laboratory has completed the applicable EHRS lab safety training.
Training programs are developed and presented by the Office of Environmental Health & Radiation Safety (EHRS) using instructor led or web-based formats. As of March 6, 2023, Workday Learning is replacing Penn Profiler and Knowledge Link. If you have been assigned a training curriculum prior to March 3, 2023, you will not have to make new training assignments in Workday Learning.
On or after March 6, 2023, supervisors of new faculty, staff, and students will need to assign EHRS training through Workday Learning. To determine which job-related training programs you are required to assign, use the EHRS Workday Learning Selection Guide for your campus and job description. Penn employees can also self-assign courses offered by EHRS. For more information, see our Training website.
All Introduction to Laboratory Safety Training courses cover the following information about Penn’s Chemical Hygiene Plan:
- The contents of the OSHA standard 29 CFR 1910.1450 and its appendices. These are available to employees from Environmental Health and Radiation Safety and from www.osha.gov.
- The location and availability of the University of Pennsylvania's Chemical Hygiene Plan.
- The location and availability of known reference material on the hazards, safe handling, storage and disposal of hazardous chemicals found in the laboratory. This may include Safety Data Sheets and other reference sources.
- The existence of Standard Operating Procedures and Fact Sheets and their applicability to the laboratory.
- The existence of a Chemical Hygiene Work Plan and its applicability to the laboratory.
- Emergency response procedures for lab incidents.
Additional task-specific training is the responsibility of the Principal Investigator and must be as specific to the activities conducted in the laboratory as possible. It must include:
- The identity of any specific hazardous materials, equipment, or processes that the student or lab worker is expected to encounter in their research or is part of the research activities of others in the lab
- The specific health and safety risks associated with those materials, equipment, and processes
- For each hazardous material, equipment, and process: (a) The required engineering controls for safe handling and use, (b) The required work practices for safe handling and use, (c) The required personal protective equipment for safe handling and use
- The availability and location of personal protective equipment (PPE)
- Instruction on proper operation of the fume hood and other engineering controls in the lab
- The location and function of emergency irrigation equipment, fire alarms pull stations, and emergency exits
- The consequences of non-compliance with university or laboratory safety policies.
- The permissible exposure limits for OSHA regulated substances (or published exposure limits for hazardous chemicals where there is no applicable OSHA standard) for chemicals used in their lab. (available from EHRS)
- Signs and symptoms associated with exposures to hazardous chemicals used in their laboratory. (available from EHRS)
- Health risks (both chemical and physical) posed by the experimental procedures conducted in their lab.
- The existence and location of all designated areas in the laboratory.
- The selection and use of personal protective equipment appropriate for laboratory tasks. See Section XV for additional information on personal protective equipment.
Revised 10/2017
Exposure to some chemicals can result in acute or chronic health effects. Other chemicals may have properties which make them physically hazardous. It is also possible for a single substance to exhibit a combination of health hazards and physical hazards.
Various health and physical hazards of chemicals are described below.
Chemical Health Hazards
Chemical Health Hazards
Health-hazardous chemicals are chemicals for which there is statistically significant evidence (based on at least one study conducted according to established scientific principles), that acute or chronic health effects may occur in exposed employees, or if it is listed in any of the following:
- OSHA, 29 CFR 1910 Subpart Z, Toxic and Hazardous Substances
- "Threshold Limit Values for Chemical Substances and Physical Agents in the Work Environment", ACGIH (latest edition)
- "The Registry of Toxic Effects of Chemical Substances", NIOSH (latest edition)
- 29 CFR 1910.1048 Occupational Exposure to Formaldehyde
In most cases, the label will indicate if the chemical is hazardous. Look for key words like caution, hazardous, toxic, dangerous, corrosive, irritant, or carcinogen. Old containers of hazardous chemicals (pre 1985) may not contain hazard warnings.
If you are not sure that a chemical you are using is hazardous, review the Safety Data Sheet (SDS) or contact your supervisor, instructor, or the Office of Environmental Health and Radiation Safety.
The following Chemical Health Hazards are defined below:
- Irritants
- Asphyxiants
- Anesthetics
- Hepatoxins
- Nephrotoxins
- Neurotoxins
- Hematopoietic Toxins
- Inorganic Dusts
- Carcinogens
- Reproductive Hazards
- Sensitizers
- Acutely Toxic Chemicals
Chemical Toxicity
Toxicology is the study of the nature and action of poisons. Toxicity is the ability of a chemical molecule or compound to produce injury once it reaches a susceptible site in or on the body. Toxicity hazard is the probability that injury will occur considering the manner in which the substance is used.
Dose–Response Relationships
The potential toxicity (harmful action) inherent in a substance is manifest only when that substance comes in contact with a living biological system. A chemical normally thought of as "harmless" will evoke a toxic response if added to a biological system in sufficient amount. The toxic potency of a chemical is defined by the relationship between the dose (the amount) of the chemical and the response that is produced in a biological system.
Routes of Entry into the Body
There are three main routes by which hazardous chemicals enter the body:
- Absorption through the respiratory tract through inhalation. This is most important in terms of severity.
- Absorption or Injection through the skin or eyes.
- Absorption through the digestive tract through ingestion. This can occur through eating or smoking with contaminated hands or in contaminated work areas.
Most exposure standards including ACGIH Threshold Limit Values (TLVs) and OSHA Permissible Exposure Limits (PELs), are based on the inhalation route of exposure. They are normally expressed in terms of either parts per million (ppm) or milligrams per cubic meter (mg/m3) concentration in air.
If a significant route of exposure for a substance is through skin contact, the SDS will have a "skin" notation. Examples include: pesticides, carbon disulfide, phenol, carbon tetrachloride, dioxane, mercury, thallium compounds, xylene, hydrogen cyanide.
Health Effects
Acute poisoning is characterized by rapid absorption of the substance and the exposure is sudden and severe. Normally, a single large exposure is involved. Examples: carbon monoxide or cyanide poisoning.
Chronic poisoning is characterized by prolonged or repeated exposures of a duration measured in days, months or years. Symptoms may not be immediately apparent. Examples: lead or mercury poisoning and pesticide exposure.
Local refers to the site of action of an agent and means the action takes place at the point or area of contact. The site may be skin, mucous membranes, the respiratory tract, gastrointestinal system, eyes, etc. Absorption does not necessarily occur. Examples: strong acids or alkalis.
Systemic refers to a site of action other than the point of contact and presupposes absorption has taken place. For example, an inhaled material may act on the liver. Examples: arsenic affects the blood, nervous system, liver, kidneys and skin; benzene affects the bone marrow.
Cumulative poisons are characterized by materials that tend to build up in the body as a result of numerous chronic exposures. The effects are not seen until a critical body burden is reached. Examples: heavy metals.
Synergistic responses: When two or more hazardous material exposures occur the resulting effect can be greater than the effect of the individual exposures. This is called a synergistic or potentiating effect. Example: exposure to both alcohol and chlorinated solvents.
Chemicals dissolved in dimethyl sulfoxide (DMSO) pose a serious skin absorption hazard. DMSO greatly increases the transport of solute through the skin.
Other Factors Affecting Toxicity
Rate of entry and route of exposure; that is, how fast is the toxic dose delivered and by what means. Age can affect the capacity to repair tissue damage. Previous exposures can lead to tolerance, increased sensitivity or make no difference.
State of health, physical condition, and life style, can affect the toxic response. Preexisting disease can result in increased sensitivity.
Environmental factors such as temperature and pressure may also affect the exposed individual as well as host factors including genetic predisposition and the sex of the exposed individual.

Irritants
Irritants are materials that cause inflammation of the body surface with which they come in contact.
The inflammation results from concentrations far below those needed to cause corrosion.
See the Chemical Hygiene Plan SOP for Irritants for more information about health effects and safe handling practices for irritant chemicals.
Common irritants include substances such as:
- ammonia
- alkaline dusts and mists
- hydrogen chloride
- hydrogen fluoride*
- halogens
- ozone
- phosgene*
- nitrogen dioxide
- phosphorus chloride
- arsenic trichloride
* these materials also have other hazardous properties.
Irritants can also cause changes in the mechanics of respiration and lung function. These include:
- sulfur dioxide
- acetic acid
- formaldehyde*
- formic acid
- sulfuric acid
- acrolein
- halogens
* these materials also have other hazardous properties.
Long term exposure to irritants can result in increased mucous secretions and chronic bronchitis.
A primary irritant exerts no systemic toxic action, either because the products formed on the tissue of the respiratory tract are non-toxic or because the irritant action is more severe than any systemic toxic action. Example: hydrogen chloride.
A secondary irritant's effect on mucous membranes is overshadowed by a systemic effect resulting from absorption. These include:
- hydrogen sulfide
- aromatic hydrocarbons
Exposure to a secondary irritant can result in pulmonary edema, hemorrhage and tissue necrosis.
Sensitizers
A sensitizer causes a majority of the exposed population to develop an allergic reaction in normal tissue after repeated exposure to the chemical.
The reaction may be as mild as a rash (contact dermatitis) or as serious as anaphylactic shock.

Carcinogens
The term carcinogen describes any agent that can initiate or speed the development of malignant or potentially malignant tumors, malignant neoplastic proliferation of cells, or cells that possess such material.
A list of carcinogenic materials can be found in the SOP for Handling Carcinogens in this CHP.
Carcinogens commonly used in large quantities at the University include formaldehyde, benzene, ethylene amine, ethylene oxide, and chloroform.
A select carcinogen is any substance that meets one of the following criteria:
- It is regulated by OSHA as a carcinogen
- It is listed under the category, "known to be carcinogens" in the National Toxicology Program (NTP), "Annual Report of Carcinogens" (latest edition)
- It is listed under Group 1, "carcinogenic to humans" by the International Agency for Research on Cancer Monographs (IARC)
- It is listed under Group 2A or 2B by IARC or under the category "reasonably anticipated to be carcinogens" by NTP, and causes statistically significant tumor incidence in experimental animals according to any of the following criteria:
- After inhalation exposure of 6-7 hours per day, 5 days per week, for a significant portion of a lifetime, to doses of less than 10 mg/m3
- After repeated skin application of 300 mg/kg of body weight per week
- After oral doses of less than 50 mg/kg of body weight per day
Reproductive Hazards
Reproductive hazards are chemicals that affect the reproductive capabilities including chromosomal damage (mutagens) and effects on the fetus (teratogens).
See the Chemical Hygiene Plan SOP for Reproductive Hazards for more information about health effects, safe handling practices, and a list of chemicals that are hazardous to the reproductive system.
- A mutagen affects the chromosome chains of exposed cells. The effect is hereditary and becomes part of the genetic pool passed on to future generation.
- A teratogen (embryotoxic or fetotoxic agent) is an agent that interferes with normal embryonic development and may lead to birth defects or even death. Effects are not hereditary.

Toxic Chemical Classes
Hepatotoxic
Hepatotoxic agents cause damage to the liver.
These include:
- carbon tetrachloride
- tetrachloroethane
- nitrosamines
Nephrotoxins
Nephrotoxic agents damage the kidneys.
These include:
- halogenated hydrocarbons
- uranium compounds
Neurotoxins
Neurotoxic agents damage the nervous system.
The nervous system is especially sensitive to organometallic compounds and certain sulfide compounds.
These include:
- trialkyl tin compounds
- tetraethyl lead
- methyl mercury
- carbon disulfide
- organic phosphorus insecticides
- manganese
- thallium
Hematopoietic Toxins
Some toxic agents act on the blood or hematopoietic system.
The blood cells can be directly affected or the bone marrow can be damaged.
These include:
- nitrites
- aniline
- toluidine
- nitrobenzene
- benzene
Acutely Toxic Chemicals
Acutely toxic chemicals are substances falling into the following categories:
See the Chemical Hygiene Plan SOP for Acutely Toxic Chemicals for more information about health effects, safe handling practices, and a list of acutely toxic chemicals.
- A chemical that has a median lethal dose (LD50) of 50 milligrams or less per kilogram of body weight, when administered to albino rats weighing 200g to 300g each.
- A chemical that has a median lethal dose (LD50) of 2000 milligrams or less per kilogram of body weight, when administered by continuous contact for 24 hours, (or less if death occurs within 24 hours), to the bare skin of albino rabbits weighing 200g to 300g each.
- A chemical that has a median lethal concentration (LC50) in air of 200 parts per million by volume, or less, of gas, or vapor, or 2 milligrams per liter or less, of mist, fume, or dust, when administered by continuous inhalation for one hour, (or less if death occurs within one hour), to albino rats weighing 200g to 300g each.
Extremely toxic chemicals are substances that cause irreversible neurological damage or death with extremely small doses.
Substances in this class include many organic mercury compounds such as dimethyl mercury and MPTP (1-methyl-4phenyl-1, 2, 3, 6-tetrahydropyridine) which can cause irreversible Parkinsonian syndrome. Lab work with these materials requires review by EHRS and typically includes chemical resistant gloves and protective clothing.
Asphyxiants
Simple Asphyxiants deprive the tissue of oxygen. Simple asphyxiants are inert gases that displace oxygen.
These include:
- nitrogen
- nitrous oxide
- carbon dioxide
- helium
Chemical asphyxiants render the body incapable of maintaining an adequate oxygen supply.
They are active at very low concentrations (few ppm).
These include:
- carbon monoxide
- cyanides
Anesthetics
Primary anesthetics have a depressant effect upon the central nervous system, particularly the brain.
These include:
- halogenated hydrocarbons
- alcohols
Inorganic Dusts
There are toxic agents that produce damage of the pulmonary tissue (lungs) but not by immediate irritant action.
Fibrotic changes can be caused by free silica and asbestos. Other dusts can cause a restrictive disease called pneumoconiosis.
Physical Hazards of Chemicals
Physical Hazards
OSHA defines a physical hazard as a chemical for which there is scientifically valid evidence that it is a combustible liquid, a compressed gas, explosive, flammable, an organic peroxide, an oxidizer, pyrophoric, unstable (reactive), or water-reactive.
The following Physical Hazards of Chemicals are defined below:
- Combustible and Flammable Liquids
- Compressed Gases
- Corrosive Chemicals
- Reactive Chemicals
- Explosives
- Organic Peroxides
- Oxidizers
- Pyrophorics
- Water Reactives

Flammable Liquids
Flammable and combustible liquids are those liquids which can form a vapor/air mixture that is capable burning in the presence of an ignition source.
In general, flammable liquids will ignite much more easily than combustible liquids.
See the Chemical Hygiene Plan SOP for Flammable Liquids for more information about handling and storage practices for combustible and flammable liquids.
The flashpoint of a flammable liquid is the lowest temperature at which it can form an ignitable mixture with air and produce a flame when a source of ignition is present.
Combustible liquids have a flashpoint at or above 37.8°C (100°F) and below 93.3°C (200°F). Combustible liquids are divided into three classes:
| Class | Flash Point | Example |
| II | 100-139°F | Acetic acid, naptha and stoddard solvent |
| IIIA | 140-199°F | Cyclohexanol, formic acid and nitrobenzene |
| IIIB | 200°F or above | Formalin and picric acid |
Flammable liquids are chemicals that have a flash point below 100°F (38.7°C) and a vapor pressure that does not exceed 40 psig at 100°F. Flammable liquids are divided into three classes:
|
Class |
Flash Point |
Boiling Point | Example |
| IA | Below 73°F | Below 100°F | Ethyl Ether |
| IB | Below 73°F | At or above 100°F | Acetone, Benzene, Toluene |
| IC | At or above 73°F and below 100°F | Hydrazine and Styrene |
Source: National Fire Protection Association (NFPA)
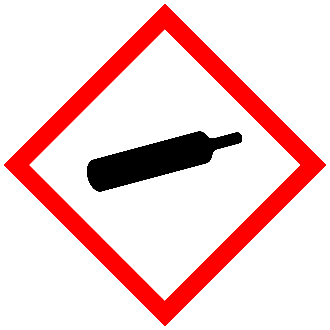
Compressed Gases
All compressed gases pose a physical hazard because of the high pressures inside the cylinders.
See the Chemical Hygiene Plan SOP for Compressed Gases for more information about handling and storage practices for gases under pressure.
There have been many cases in which damaged cylinders have become uncontrolled rockets or pinwheels and have caused severe injury and damage. This danger has happened when unsecured, uncapped cylinders were knocked over causing the cylinder valve to break and high pressure gas to escape rapidly.
Poorly controlled release of compressed gas in chemical reaction systems can cause vessels to burst, create leaks in equipment or hoses, or produce runaway reactions.
Compressed gases can be either liquefied, non-liquefied, or dissolved.
[source: https://www.ccohs.ca/oshanswers/chemicals/compressed/compress.html]
Depending on the identity of the compressed gas, there may also be additional hazards such as fire, explosion, corrosion, asphyxiation, and toxicity.
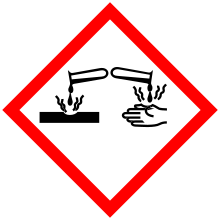
Corrosive chemicals are highly reactive substances that cause obvious damage to living tissue.
See the Chemical Hygiene Plan SOP for Corrosives for more information about safe handling and storage practices.
Corrosives act either directly, by chemically destroying the part (oxidation), or indirectly by causing inflammation. Acids and bases are common corrosive materials.
Corrosive chemicals may also be referred to as "caustic", although the term caustic usually applies to strong bases.
Reactive Chemicals
The Chemical Hygiene Plan SOP Reactive Chemicals addresses the safe storage and handling of highly reactive materials which meet one or more of the following criteria:
- undergo vigorous polymerization, condensation or decomposition
- become self-reactive under conditions of shock or increase in pressure or temperature
- react vigorously with water to release a lethal gas
Many "highly reactive" chemicals are categorized as one of the following more-specific reactivity classes:
Explosive (and potentially explosive) Compounds SOP
Be sure to review the SOP or SOPs that best addresses the hazard type of your chemical. Contact EHRS if you have any questions about which requirements apply.
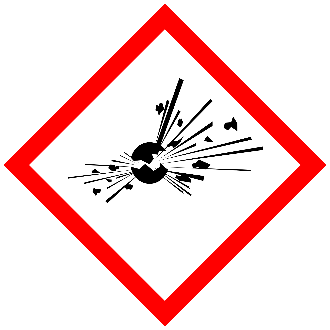
Explosives
An explosive is any chemical compound or mechanical mixture that, when subjected to heat, impact, friction, detonation, or other suitable initiation, undergoes rapid chemical change, evolving large volumes of highly heated gases—typically nitrogen or CO2—that exert pressure on the surrounding medium.
The term applies to materials that either detonate or deflagrate
[Source: Prudent Practices in the Laboratory: Handling and Management of Chemical Hazards (section 4.D.3.1 Explosive Hazards) The National Academies Press: Washington, DC, 2011.]
See the Chemical Hygiene Plan SOP for Explosive Compounds for more information about safe handling and storage practices, and identifying explosives and potentially explosive compounds.
Organic Peroxides
Organic peroxides can be severe fire and explosion hazards.
An organic peroxide is any organic (carbon-containing) compound having two oxygen atoms joined together (-O-O-). This chemical group is called a "peroxy" group.
[Source: https://www.ccohs.ca/oshanswers/chemicals/organic/organic_peroxide.html]
Examples of types organic peroxides include:
- dialkyl peroxides
- hydroperoxides
- diacyl peroxides
- peroxydicarbonates
- peroxyesters
- ketone peroxides
- peroxyketals
- alkylperoxy carbonates
See the following Chemical Hygiene Plan SOPs for more information about safe handling, storage, and hazards of organic peroxides:
Depending on the identity of the organic peroxide, there may also be additional hazards such as flammability, corrosion, and toxicity.
Organic peroxides may also have a self-accelerating decomposition temperature (SADT). SADT represents the lowest temperature in which that particular organic peroxide formulation in its commercial packaging will undergo self-accelerating decomposition (begin the chemical process that leads to explosion). The SADT value will vary with each organic peroxide formulation and the size and shape of its packaging. Storage requirements will generally be 10 to 20 degrees below the SADT.
[Source: https://www.ccohs.ca/oshanswers/chemicals/organic/organic_peroxide.html]
Oxidizers
Oxidizing chemicals are materials that spontaneously evolve oxygen at room temperature (or with slight heating) or chemicals that promote combustion.
This class of chemicals includes peroxides, chlorates, perchlorates, nitrates, and permanganates. Strong oxidizers are capable of forming explosive mixtures when mixed with combustible, organic or easily oxidized materials.
See the Chemical Hygiene Plan SOP for Strong Oxidizers for more information about safe handling and storage practices. Also see the Chemical Hygiene Plan Fact Sheet for Gas-Producing Waste for special procedures for waste streams containing hydrogen peroxide and other strong oxidizers.
Examples of strong oxidizers include:
|
Ammonium perchlorate |
Ammonium permanganate |
[Source: CRC Handbook of Laboratory Safety, 3rd edition.]
Pyrophorics
OSHA defines a pyrophoric material as a liquid or solid which, even in small quantities, is liable to ignite within five minutes after coming into contact with air, at or below 55 °C (130 °F).
Many pyrophoric materials are also water-reactive, reacting vigorously with water or high humidity, often igniting upon contact.
See the Chemical Hygiene Plan SOP for Pyrophoric Chemicals for more information about safe handling and storage practices.
Examples of pyrophoric materials include:
Liquids
Organolithiums
- Alkyl and Aryl Lithiums
- n-butyllithium,
- t-butlylithium
- Lithium Amides
- Lithium Alkoxides
Organomagnesiums “Grignard Reagents”
- Alkyl and Aryl Magnesium Halides
- Methylmagnesium Chloride, Allylmagnesium Bromide
Organozincs
- Diethyl Zinc
Aluminum Alkyls
- Trimethylaluminum
- Diisobutylaluminum hydride
Metal Carbonyls
- Nickel Carbonyl
- Iron Pentacarbonyl
Silicon Halides
- Dichloromethylsilane
Solids
Metal Hydrides
- Sodium Hydride
- Potassium Hydride
- Lithium Aluminum Hydride
Finely Divided Metals
- Aluminum
- Lithium
- Magnesium
- Titanium
- Zinc
- Zirconium
- Sodium
- Potassium
Used Hydrogenation Catalysts
- Raney Nickel
- Palladium on Carbons
Gases
- Silane
- Diborane
- Phosphine
Water-Reactive Chemicals
Water-Reactive chemicals are chemicals that react vigorously with moisture.
See the Chemical Hygiene Plan SOP for Water-Reactive Chemicals for more information about safe handling and storage practices.
The most common water-reactive chemicals include sodium, potassium, lithium metals and aluminum alkyls.
Water-reactive chemicals are sometimes pyrophoric and the handling and storage procedures in the Chemical Hygiene Plan SOP for Pyrophoric Chemicals would apply.
Revised 10/2021
A label is any written, printed, or graphic material displayed on, or affixed to, containers of chemicals.
Labels or other forms of hazard warnings, such as tags or placards, provide immediate warning of potential danger. They are used to warn of a variety of potential physical hazards, or health hazards.
All hazardous chemicals throughout Penn’s campus must be properly labeled. The existing manufacturer label must not be removed, altered or defaced. If the original label must be replaced, it must contain the same information as the original label. Labels, as required by the Occupational Safety and Health Act (OSHA) Hazard Communication standard 1910.1200, must contain the following information:
- Manufacturer name, address and phone number
- Product identifier
- Signal word (“Warning”, “Danger”)
- Hazard statement(s)
- Pictogram(s) (https://www.osha.gov/Publications/HazComm_QuickCard_Pictogram.html)
- Precautionary statement(s)
Carefully read all the information on the label. If you do not understand something, contact your supervisor or instructor for an explanation or request the SDS.
This labeling requirement does not apply to students assigned unknown chemicals for analysis. However, hazard information must be provided for all unlabeled chemicals in student laboratories.
If commercially-acquired chemicals are transferred out of the original container, the new container must be labeled with the full chemical name or other non-ambiguous identifier. It is a best practice to include the date prepared and the owner's name on the label.
If a chemical container is reused to contain a different material, the original label must be removed or thoroughly defaced to avoid confusion about the contents.
Labels must be written legibly and replaced when damaged or faded.
All containers of hazardous materials 4-L or larger must be labeled with both the name and the chemical hazards.
Chemical hazard labels must be affixed to or written on any semi-permanent container of hazardous materials in the lab such as stock solutions and acid and base baths. It is highly recommended that pre-labeled bottles are purchased for solvents transferred to squeeze-type wash bottles.
Labels for laboratory containers of hazardous material can be requested from EHRS using this webform: https://ehrs.upenn.edu/health-safety/health-safety-forms/warning-sign-and-label-request-form.
If a researcher transfers hazardous chemicals from a labeled container to a portable container that is only intended for immediate use by the researcher who performs the transfer (e.g. a reaction flask or beaker), no labels are required for the portable container.
Containers of non-hazardous materials (e.g. water or buffers) must be legibly labeled with the name of all contents. Abbreviations, acronyms, and common trade names can be used if a list is posted nearby with definitions. Spills and leaks of improperly labeled, non-hazardous materials have resulted in inappropriate emergency after-hours response because the material could not be identified by first responders.
All containers of non-hazardous materials 4-L or larger must be labeled with the words “non-hazardous.” Non-hazardous labels can be requested from EHRS using this webform: https://ehrs.upenn.edu/health-safety/health-safety-forms/warning-sign-and-label-request-form.
10/26/2021, G. Allen
All containers that hold carcinogens, reproductive hazards or acutely toxic reagents must be properly labeled concerning the health hazard posed by the chemical. Most newer reagent containers will have the chemicals hazard clearly displayed on the label. However older reagents and containers of solutions that are mixed in the lab must be properly labeled by the laboratory worker.
Acid and Base Baths should be labeled with the OSHA hazard pictogram and contents. EHRS has templates for the bath labels to use with Avery UltraDuty GHS Chemical Labels 60502.
Revised 1/2022
General Guidance
Before using a chemical for the first time or when using it in novel way, review the properties of each material that will be used. Consider both the health hazards and the physical hazards of the chemical, including any reactivity or chemical incompatibility hazards. Sources of chemical safety information for particular substances include the material’s Safety Data Sheet (SDS), available from the chemical manufacturer or EHRS, and Laboratory Chemical Safety Summaries (LCSSs), available from PubChem. See the Hazardous Chemicals section of this Chemical Hygiene Plan for explanations of the various chemical hazard types. When you have identified all of the physical and chemical health hazards associated with your procedure, review the appropriate Standard Operating Procedures for each of the hazards.
For each experiment, consider the hazards that may result when chemicals are mixed together or exposed to conditions such as elevated temperature or pressure. Those conditional hazards may not be evident in the SDS or LCSS. The properties of each chemical must be considered in order to understand how it may behave during use. Resources such as Bretherick's Handbook of Reactive Chemical Hazards (available online through Penn Library) and Cameo Chemicals (free online tool) are useful resources for evaluating chemical incompatibilities and reactivity hazards.
If you are using a new chemical that introduces a novel hazard to your lab, or if the risks of working with that chemical are higher than what you would normally handle in your daily tasks, then a Hazard Control Plan must be written to describe the risks and controls for the experiment.
The following minimum guidance must be followed for all chemical handling tasks. Additional guidance for working with chemicals based on their hazard type is found in the Standard Operating Procedures:
- Minimize the quantity of hazardous chemicals that are ordered and handled in the laboratory as much as is possible.
- Substitute hazardous chemicals with less-hazardous alternatives as much as possible.
- Do not work alone in the laboratory when handling hazardous materials of any quantity.
- Wear required personal protective equipment including lab coat, safety glasses, and gloves, plus any additional protective equipment prescribed by the relevant SOP or Hazard Control Plan.
- Label all containers with the full name of all chemical contents unless you will be using the chemical immediately in your process.
- Wash hands thoroughly with soap and water after handling any chemical and whenever you leave the lab.
- Use good housekeeping practices to avoid contamination of surfaces, garments, personal belongings, and self.
- Never intentionally smell, inhale or taste a chemical.
- Smoking, drinking, eating, and the application of cosmetics is forbidden in areas where hazardous chemicals are used or stored.
- Open containers of hazardous chemicals may only be handled inside of a properly functioning fume hood unless the process has been evaluated by EHRS.
- Inspect equipment or apparatus for damage before adding a hazardous chemical. Do not use damaged equipment.
- Never use mouth suction to fill a pipette. Use a pipette bulb or another pipette-filling device. (See the Biosafety Manual for more on pipetting.)
- Promptly clean-up spills if you are able to do so safely. Contact EHRS for assistance with spill clean-up when necessary. Make sure your work space is free of chemical contamination when you are finished working.
Controlling the Risks of Chemical Hazards
The specific methods for controlling chemical hazards are described in the Standard Operating Procedures for each chemical hazard type and are further defined by the Hazard Control Plan for high hazard experiments. The following general principles of hazard control are used to determine those guidelines:
- Elimination of hazards or substitution with lower risk materials or processes
- Engineering controls protect the researcher by isolating the hazard at the source
- Administrative controls protect the researcher through work practices that lower the risks of performing the task
The controls above prevent a dangerous condition from occurring. The last level of the hierarchy of controls (PPE) prevents injury when those methods are not able to provide adequate protection.
- Personal Protective Equipment (PPE) is used as last line of defense against exposure or injury in case the other control measures are insufficent
(More information about the hierarchy of controls is available at: https://www.cdc.gov/niosh/topics/hierarchy/)
Whenever it is feasible to do so, avoid using hazardous chemicals.
When it is necessary to work with hazardous materials and processes, the following controls must be considered first:
- Substitution of a less hazardous substance
- Substitution of less hazardous equipment or process (e.g., safety cans for glass bottles)
- Isolation of the operator or the process (e.g. use of a fume hood or glove box)
When lab hazards cannot be eliminated, engineering controls should be the first type of control considered for reducing risk.
The National Institute of Occupational Safety and Health (NIOSH) defines engineering controls as a control method “designed to remove the hazard at the source, before it comes in contact with the worker. Well-designed engineering controls can be highly effective in protecting workers and will typically be independent of worker interactions to provide this high level of protection.” Engineering controls should be the first type of control considered for reducing the risks of lab hazards.
Chemical fume hoods are one example of an engineering control and these are discussed in more detail below. Other examples of engineering controls used to manage laboratory risks include:
- Blast shielding
- Glove box
- Machine guarding
- Liquid traps
- Vacuum filtration
Reducing risk through engineering controls also means selecting the equipment, apparatus, and lab ware that are appropriate for the materials and hazards of your work.
For example:
When performing small-volume liquid transfers (<10 mL) of highly hazardous materials (toxic, corrosive, or air-sensitive), it is not appropriate to use a luer-slip syringe type, because the needle can easily detach from the syringe barrel. Luer-lock or integrated-needle syringes must be used with these chemicals.
When performing larger-volume liquid transfers (>10 mL) of highly hazardous materials, a double ended needle (aka: cannula) must be used, to further reduce the risks posed by syringes, such as plunger ejection.
These are just a few examples of engineering controls. The specific methods for controlling chemical hazards are described in the Standard Operating Procedures for each chemical hazard type and are further defined by the Hazard Control Plan for high hazard experiments.
Local exhaust ventilation is one of the best engineering methods available to reduce the health hazard risk associated with the use of hazardous chemicals in the laboratory.
Fume hoods are used to prevent hazardous, offensive, or flammable gases and vapors from mixing with the general room air. A hood, especially with the sash down, also acts as a physical barrier between the laboratory workers and chemical reactions. The hood can also contain accidental spills of chemicals.
Note that laboratory fume hoods and biosafety cabinets, although similar in appearance, are extremely different devices. Biosafety cabinets are used for protection against exposure to biological materials and should not be used with chemicals unless specifically designed for this purpose. If you are uncertain about the type of hood or biosafety cabinet in your laboratory, check with your Principal Investigator or EHRS.
Check the SDS, appropriate Standard Operating Procedure, or chemical label for special ventilation requirements, such as:
- Use with adequate ventilation
- Use in a fume hood
- Avoid inhalation of vapors
- Provide local ventilation
If a fume hood is not available in your work space, contact EHRS before working with any hazardous chemicals.
To be effective, laboratory fume hoods must be installed and used correctly. The National Research Council in Prudent Practices for Handling Hazardous Chemicals in Laboratories; (1981) recommends that the following factors be remembered in the daily use of hoods:
- Hoods should be considered as backup safety devices that can contain and exhaust toxic, offensive, or flammable materials, when the design of an experiment fails. Hoods should not be used as a means for disposing of chemicals. Thus, apparatus used in hoods should be fitted with condensers, traps, or scrubbers to contain and collect waste solvents or toxic vapors or dusts.
- Hoods should be evaluated before use to ensure adequate face velocities (typically 80-100 fpm) and the absence of excessive turbulence. Further, some continuous monitoring device for adequate hood performance should be present and should be checked before each hood is used. If inadequate hood performance is suspected, it should be established that the hood is performing adequately before it is used. Call your building administrator to report inoperable hoods.
- Except when adjustments of apparatus within the hood are being made, the hood should be kept closed: vertical sashes down and horizontal sashes closed. Sliding sashes should not be removed from horizontal sliding-sash hoods. Keeping the face opening of the hood small improves the overall performance of the hood.
- The airflow pattern, and thus the performance of a hood, depends on such factors as placement of equipment in the hood, room drafts from open doors or windows, persons walking by, or even the presence of the user in front of the hood. For example, the placement of equipment in the hood can have a dramatic effect on its performance. Moving an apparatus 5-10 cm back from the front edge into the hood can reduce the vapor concentration at the user's face by 90%. Elevate large pieces of equipment 2-3 inches off of the work surface.
- Hoods are not intended for storage of chemicals. Materials stored in them should be kept to a minimum. Items stored in the hoods must not block vents or alter airflow patterns. Whenever possible, chemicals should be moved from hoods into cabinets for storage.
- Solid objects and materials (such as paper) should not be permitted to enter the exhaust ducts of hoods as they can lodge in the ducts or fans and adversely affect their operation.
- An emergency plan should always be prepared for the event of ventilation failure (power failure, for example) or other unexpected occurrence such as fire or explosion in the hood.
At Penn some hoods fume hoods are equipped with combination sashes, which include both a vertical-moving sash and also horizontal-sliding panels. These hoods are meant to be used in one of two ways:
1) With the vertical-moving sash raised to a position that is high enough to allow the researcher access to the materials in the hood, but low enough that the sash is protecting the researcher’s face and upper body.
2) With the vertical-moving sash completely down and one of the horizontal-sliding sash panels positioned directly in front of the researcher’s body. In this way, the researcher’s arms can reach around the panel to access the interior of the hood, while their entire body is shielded by the panel in front of them.
Fume Hood Inspection Program
The function of each fume hood on campus is tested upon initial installation. The velocity of the air at the face of the hood is also confirmed by EHRS annually and after any repairs are made to the fume hood fans or alarms. EHRS tests the air flow with the hood sash positioned at the height of the arrow on the green sticker along the side of the face of the hood. The hood should not be used for work with hazardous materials unless the sash is at or below this indicator. More information about the fume hood inspection program can be found on the Fume Hood website.
Reporting Fume Hood Problems
Check your hood before each use. If the alarms sounds or if you detect a problem, close your hood sash completely and re-open it no higher than the level of the arrow on the green sticker located along the side of the face of the hood.
If low-flow or no-flow is still detected and/or the alarm is sounding, follow these steps:
1) Stop all work with hazardous materials.
2) Cap all open containers in the hood and de-energize any equipment such as pumps and hot plates.
3) Contact the building administrator to request repair.
Under no conditions is it acceptable to work with hazardous materials in a fume hood that is not functioning properly. Even if you can feel/hear air flowing in the hood, you may not work in hood while the alarm is indicating low or no flow.
The building administrator will place an orange Hood Out of Order sign on the sash of the hood.
A mechanic will evaluate the hood and make the necessary repairs. Once the hood is repaired, Environmental Health and Radiation Safety will test the flow. If the hood's face velocity is adequate, the orange sign will be removed. Do not resume use of the fume hood until EHRS has confirmed adequate face velocity. Report to EHRS persistent problems with fume hoods or repair delays longer than 5 working days.
For more information on inspection and repair procedures, including building specific procedures, see Fume Hoods.
Administrative controls are work practices that reduce the risks associated with hazardous processes.
Because administrative controls rely on training and worker compliance, these methods are often used in addition to engineering controls in order to provide a higher level of risk reduction. Some examples of administrative controls in the laboratory include:
General laboratory practices such as
- Proper labeling and storage
- Good laboratory housekeeping
- Not working alone in the lab
- Testing equipment function and inspecting for damage prior to use
One example of a process-specific administrative control would be: Frequently venting a separatory funnel during a liquid-liquid extraction, and pointing the stopcock outlet away from oneself when venting.
The use of Designated Areas to segregate hazardous and non-hazardous work is also an administrative control for reducing risk of exposure or injury. See Section VI: Chemical Storage and Transportation in this CHP for more information about Designated Area requirements.
These are just a few examples of administrative controls. The specific methods for controlling chemical hazards are described in the Standard Operating Procedures for each chemical hazard type and are further defined by the Hazard Control Plan for high hazard experiments.
Personal Protective Equipment (PPE)
Revised 3/2022
If something unexpected occurs or the engineering and administrative controls are not sufficient, then the researcher's Personal Protective Equipment (PPE) provides a last line of defense against injury.
Personal Protective Equipment (PPE) is the term used for all wearable protective equipment such as safety glasses, lab coats, gloves, face shields, chemical aprons, sleeve protectors, respirators, etc.
The PI is responsible for enforcing the use of personal protective equipment in his or her laboratory.
The standard attire required in all laboratories is long pants and enclosed shoes. Shorts, skirts, sandals and other garments or footwear that leave skin exposed below the lab coat are not permitted to be worn in the laboratory.
Standard PPE for the majority of chemical handling procedures includes a 100% cotton or fire-resistant lab coat, ANSI-approved safety glasses, and minimum 4-mil-thickness disposable nitrile gloves. Lab coats made of synthetic fibers are not permitted.
Researchers who work with liquid pyrophorics, open flame, or high volumes of flammable liquids must wear fire-resistant lab coats. Contact EHRS for more information about fire-resistant lab coats.
Additional PPE may be required for some procedures. The PI is responsible for determining when additional PPE is required in his or her laboratory based on the hazards of the work. The PI must also educate lab workers on when the additional PPE is required and enforce the proper care and use of the equipment.
Safety eye wear must be worn at all times in laboratory spaces where chemical, biological, and physical hazards are present.
Eye and face protection must be worn whenever its use will reduce or eliminate injury. Eye protection must be made available to employees, students and visitors, at no cost to them, when the potential for eye injury exists.
Areas where eye protection must be worn are: laboratories, glass-cleaning and glassblowing shops, and machine shops or any area where active or automated work with chemicals is conducted. Eye protection is required for all personnel and visitors in these areas. Everyone in the room is required to wear eye protection whenever there is any chemical in use or any experimental procedure is in progress except when working at write-up station. No personnel without eye protection may enter laboratories where chemicals are being handled or automated processes are in operation.
Ordinary (street) prescription glasses do not provide adequate protection. (These glasses cannot pass the rigorous test for industrial safety glasses.) Adequate safety glasses must meet the requirements of the standard Practice for Occupational and Educational Eye and Face Protection (ANSI Z.87.1 1989) and must be equipped with side shields.
Safety glasses with side shields do not provide adequate protection from splashes, therefore, when the potential for a splash hazard exists other eye protection and/or face protection must be worn.
Splash goggles (acid goggles) with splash proof sides or a face shield must be used when protection from a liquid splash is needed.
Face shields afford protection to the face and neck. A face shield must be worn if there is an explosion or implosion (pressure or vacuum) hazard, when transferring cryogenic liquids, and in other situations where a splash, aerosol, or flying particles are likely.
Special eye protection is available for protection against laser, ultraviolet (UV), welding and brazing, or intense light sources.
Managers, supervisors, and principal investigators should refer to the Standard Operating Procedure in the Chemical Hygiene Plan for each hazard type or contact EHRS to determine the type(s) of eye and/or face protection necessary.If you have any questions regarding the selection of appropriate face protection, call Environmental Health and Radiation Safety at 215-898-4453.
Lab staff must wear lab coats at all times when handling chemicals or when working in areas where chemicals are stored or used. Lab coats must not be worn in common areas outside of the lab such as breakrooms, bathrooms, offices, and conference rooms.
Proper attire for entry into any lab where hazardous chemicals are used includes long pants or skirts that cover the entire leg and shoes that fully cover the toes and tops of the feet. Tight-fitting leggings are not recommended for the lab, as liquid chemicals that penetrate the garment will be held in direct contact with the skin.
Skin and body contact should not occur during routine lab operations that involve small quantities of laboratory chemicals. Any lab activity that is anticipated to result in body contact must be evaluated by EHRS.
Lab coats should not be worn outside of the lab. The employer (principal investigator) must provide lab coats and lab coat laundering services at no cost to all employees who work in the lab.
Lab coats for the majority of chemical handling procedures must be 100% cotton. Lab coats made of synthetic fibers are not permitted. Researchers who work with liquid pyrophorics, open flame, or high volumes of flammable liquids must wear fire-resistant lab coats. Contact EHRS for more information about fire-resistant lab coats.
Chemical protective clothing in the form of disposable work suits should be provided for the rare instances where body contact is anticipated or when extremely toxic chemicals are handled. Special attention must be given to sealing all openings in the clothing. Tape can be used for this purpose. Caps should be worn to protect hair from contamination. Selection of the protective clothing shall be made by EHRS.
Hand protection must be worn to protect against hazards of skin absorption of harmful substances, biological agents, radioactive materials, severe cuts or lacerations, severe abrasions, punctures, chemical burns, thermal burns, or harmful temperature extremes.
Chemical-Handling Gloves
Disposable nitrile gloves provide adequate protection against accidental hand contact with small quantities of most laboratory chemicals, however these gloves are not meant for protection against prolonged contact with chemicals. Lab workers who contaminate their gloves must immediately remove their gloves, wash their hands, and don new gloves. Disposable gloves may not be re-worn after they have been removed from the hands. Discard all gloves immediately after doffing.
Gloves must not be worn outside of the lab. When transporting chemicals, one glove may be worn on the hand that is carrying the secondary container, but a gloved hand must not be used on door handles or elevator buttons.
Latex gloves must not be used when working with chemicals.
More information about the selection and limitations of disposable nitrile gloves can be found in the Fact Sheet: Disposable Nitrile Gloves in Chemical Labs in this Chemical Hygiene Plan.
Lab workers should contact EHRS for advice on chemical resistant glove selection when direct or prolonged contact with hazardous chemicals is anticipated or when working with chemicals that are incompatible with nitrile. The selection of the proper glove requires knowledge of the health and physical hazards of the chemical that is used; familiarity with the glove manufacturer's test data (permeation rate and breakthrough time); and the length of the hand exposure.
Gloves for Physical-Hazard Protection
Heat-Resistant Gloves
Heat-resistant gloves have different temperature limits and intended uses. Be sure to select a glove that is designed for how you intend to use it.
Consult the glove manufacturer's product specifications or contact EHRS for assistance selecting the appropriate glove. Some examples are shown below.
Autoclave Glove

Autoclave gloves are not waterproof and should not be used for handling cryogens or dry ice. The example glove pictured is heat-resistant up to 232°C (450°F)
High-Temperature Glove

These gloves are made of cotton and have a wool liner. This material used in the example gloves pictured resists up to 2000°F (1093°C); operating temperature is 1500°F (815°C) for longer exposure. These temperatures are dependent on duration, application, and environment. Actual use temperature may be lower.
Leather Glove

Leather gloves, such as the ones shown, are generally designed for welding, and may not have a temperature rating.
Hot-glass-handling Glove

These gloves provide protection from hot glass. They may be constructed from cotton or a combination of cotton and Kevlar. The textured nitrile provides a slip-resistant surface. The heat resistance of these gloves varies. Consult manufacturer's product information.
Fire-Resistant Gloves

Lab tasks with high fire risk, such as working with pyrophoric materials or open flame, may require fire-resistant gloves that maintain good dexterity, such as Nomex pilot's gloves. You should have a Hazard Control Plan that details the required safety controls and PPE for such tasks. See also, SOP: Pyrophoric Chemicals.
Cryogen-Handling Gloves

See SOP: Cryogens and Dry Ice for information about cryogen-handling gloves.
Cut and Puncture Resistant Gloves

Puncture and cut-resistant gloves must be worn whenever there is a high risk of cut or puncture that cannot be adequately controlled through engineering and administrative controls. One example of this is when using needles that can puncture the skin and introduce an infectious agent or highly toxic substance into the body. The gloves must be rated for puncture and/or cut resistance. Do not assume that a glove is protective just because the material is thick.
Most occupational exposures do not require respiratory protection. If you think that you need a respirator contact Environmental Health & Radiation Safety. An Industrial Hygienist from EHRS will conduct a health hazard assessment to determine your potential exposure. He/She will determine if engineering controls, a change in work practices or the substitution of a less hazardous chemical can be used to reduce your exposure. If your exposure cannot be reduced, you will be provided a respirator.
If your work requires the use of a respirator, you must receive medical clearance, fit testing and training from EHRS. Never use a respirator unless you have been assigned one and have been trained and fit tested by EHRS.
Respirators are designed to protect only against specific types of substances and in certain concentration ranges, depending on the type of equipment used. Types of respiratory protective equipment include:
- Particle-removing air purifying respirators (N95, N100)
- Gas and vapor-removing air purifying respirators
- Air supplied respirators
If you would like more information about respiratory protection, please contact Valerie Perez, Industrial Hygiene Program Manager, by calling 215-898-4453 or by sending an e-mail to: Valerie Perez (vjperez@ehrs.upenn.edu).
Respirators are not to be used except in conjunction with Penn’s written respiratory protection program.
Revised 12/2020
General Considerations for Chemical Storage
Chemical storage needs must be assessed before a chemical is purchased and delivered to the laboratory. This assessment includes determining the appropriate cabinet or shelving and ensuring that sufficient space will be available. Determine chemical storage requirements by reviewing the information in this section of the Chemical Hygiene Plan and the safety data sheet for the chemical being purchased.
This section of the Chemical Hygiene Plan does not address chemical security issues. For information about securing chemicals from theft or misuse, contact EHRS.
The following general guidelines apply to all solid and liquid chemical storage areas:
- Chemicals may not be stored in the lab if:
- They have exceeded the manufacturer’s expiration date or the expiration intervals described in this section of the CHP or in any applicable SOPs.
- The container is leaking, broken, or shows signs of vapor leakage and/or chemical reaction (e.g. salt formation around cap)
- The chemical is highly hazardous and is not anticipated to be used in the next year.
All hazardous chemicals in the laboratory must be entered into Penn’s Chemical Inventory System. Information about the chemical inventory system can be found here.
All storage cabinets located in hallways and equipment corridors must be placarded with the name of the principal investigator and also with identification of the cabinet’s contents. This information is critical for emergency personnel. The lab that owns the chemical storage cabinet must complete a Chemical Storage Sign Request Form for each cabinet located in a hallway. Hazardous chemicals stored in areas outside of the laboratory must be included in the lab’s chemical inventory records (see chemical inventory section above).
Primary Storage Locations
The primary storage location for a chemical is determined by the hazards of the material. Chemical incompatibility (segregation) and chemical instability are discussed later in this section of the CHP.
Primary storage locations include:
- Refrigerator or freezer: Chemicals that must be stored at low temperature for safety or stability
- Corrosive liquids storage cabinet: Corrosive chemicals
- Flammable liquids storage cabinet: Flammable chemicals and pyrophorics
- Ventilated storage: Chemicals with strong odors and/or low odor thresholds
- Dry box or desiccator: Moisture-sensitive chemicals
- Glove box: Air-sensitive chemicals
- Open shelving or regular cabinets: Chemicals with no specified storage requirements
Chemicals that must be stored at low temperature for safety or stability can be stored in laboratory refrigerators or freezers. Flammable materials may only be stored in refrigerators/freezers if the equipment is designed for flammable material storage and UL-listed/labeled for this purpose. Contact EHRS if you are unsure whether your lab refrigerator or freezer is approved for flammable liquids storage.
Explosion-proof refrigerators/freezers are not the same as flammable-storage refrigerators/freezers. Explosion-proof devices are only required in areas where a flammable atmosphere is anticipated. In most lab situations, an explosion-proof refrigerator/freezer is not required. If you are not sure whether the model of refrigerator/freezer you wish to purchase for your lab is appropriate, please contact EHRS for guidance.
Use of household-grade refrigerators/freezers in laboratories is discouraged. Where household-grade refrigerators/freezers are used, the storage of flammable materials within them is prohibited. The refrigerator/freezer must be labeled so that it is clear that both the storage of food/drink and the storage of flammable materials are prohibited within.
When ice accumulates in a laboratory freezer, the freezer must be defrosted. The build-up of ice can cause a number of problems for chemical storage including: uneven shelf surfaces, conditions making it difficult to remove or access chemical containers, and a higher likelihood of moisture entering chemical containers. Some newer freezers are designed to prevent ice build-up, but older equipment will need to be defrosted regularly.
Hazardous chemicals must not be stored in cold rooms because cold rooms have recirculating ventilation systems. Likewise, compressed gas cylinders, liquid nitrogen dewars, and dry ice are also prohibited in cold rooms.
More information about safe use of corrosive liquids is available in the Corrosive Chemicals SOP.
Corrosive liquids must never be stored under sinks and may not be stored on shelves above eye-level.
The formation of crystals and residues around the caps of bottles of corrosive-liquids is an indication that the container is not properly sealed. Containers that show these signs of leakage must be discarded by through an EHRS chemical waste pick-up.
Inorganic corrosives
Storage cabinets that are constructed of corrosion-resistant materials are the preferred storage location for most inorganic corrosive liquids. The corrosive vapors that may escape from containers of concentrated acids and bases can damage cabinets, shelves, and brackets. This can lead to costly repairs or replacement of cabinets, and may also cause shelf failure. Thus, the storage of highly corrosive inorganic liquids in a flammable-liquids storage cabinet or in other cabinets that are not constructed of corrosion-resistant material is highly discouraged.
See the chemical segregation section below for information about the storage of acids and bases in the same cabinet.
Mildly corrosive inorganic liquids such as dilute acids and bases (1.0 N HCl or 2.0 N NaOH, for example) may be stored in open shelving. It is recommended that acids and bases stored in regular cabinets be kept on plastic trays or in plastic bins.
Amines
Amines are alkaline compounds that may be corrosive, but are generally weak bases. Amines are also commonly flammable and tend to give off strong odors. Amines do not need to be stored in a corrosion-resistant cabinet. If they are flammable, they should be kept in a flammable-liquids storage cabinet. It is usually best to store these strong-smelling chemicals in the flammable-liquids storage cabinet under the hood, as these are usually vented to the fume hood exhaust.
Organic acids and acid chlorides
Non-halogenated organic acids and acid chlorides (such as formic acid and acetic acid) are corrosive, but they are also flammable. These should be stored in a flammable-liquids storage cabinet. Keep the containers clean and tightly capped to avoid damage to the cabinet due to escaping corrosive vapor. Halogenated organic acids such as trifluoroacetic acid are non-flammable and do not need to be kept in the flammable-liquids storage cabinet.
Oxidizing acids
More information about safe use of oxidizing chemicals is available in the Strong Oxidizers SOP.
Some acids such as nitric, chromic, and sulfuric are strongly oxidizing in addition to being strongly corrosive. These acids must be kept in a corrosion-resistant cabinet and must be stored separately from all reducing agents, organic chemicals, and cellulose containing materials. Oxidizing acids must never be stored under sinks, on wooden shelves, or in wooden cabinets. They must also be kept away from paper products such as cardboard and paper towels. Strong oxidizers are highly reactive and may release hazardous gases, ignite, or form explosive mixtures on contact with wood, paper, or other organic materials.
More information about safe use of oxidizing chemicals is available in the Strong Oxidizers SOP
See “Oxidizing acids” above. This guidance applies to all strongly oxidizing chemicals.
More information about the safe use of flammable liquids is available in the Flammable Liquids SOP.
The storage of flammable and combustible liquids in a laboratory, shop, or building area must be kept to the minimum needed for research and operations. Containers one liter and larger of flammable liquids must be stored in a flammable-liquids storage cabinet. Flammable-liquids storage cabinets are not intended for the storage of compressed gases or highly corrosive chemicals. Flammable-liquids storage cabinets must meet the guidelines set forth in Lab Design & Equipment: Flammable Liquids Storage Cabinets Specifications. See also What is a Flammable Liquids Storage Cabinet?
Only compatible chemicals may be stored together inside of a single flammable-liquids storage cabinet. See “Secondary Storage Considerations: Chemical Segregation” below for details.
ChemTracker Requirements for Flammable Liquids Storage Cabinets (FLSCs)
The “Bench” location field is required for Flammable Liquids Storage Cabinets (FLSCs) in the high-rise biomedical buildings.
Flammable Liquids Storage in a Refrigerator
See “Chemical Storage in Refrigerators and Freezers” section above.
More information about the safe use of compressed gases is available in the Compressed Gases SOP and the Hazardous and Highly Toxic Gases SOP.
All compressed gas cylinders, regardless of hazard class, must be stored as follows:
- Store only the minimum amount of compressed gas required for immediate and near-term research needs. Do not stockpile gas cylinders. Promptly return unneeded gas cylinders to the vendor.
- Cylinders of hazardous compressed gases stored in common areas such as hallways must be clearly labeled with the name of the laboratory that is responsible for them.
- Cylinders must be stored in an upright position and properly secured. See Compressed Gases SOP. Compressed gas cylinders pose a crush hazard to hands and feet.
- Always use the correct regulator. Do not use a regulator adapter.
- Remove regulators when gas is not in use. If the regulator fails, the entire contents of the gas cylinder may be discharged.
- Cylinder caps must remain on the cylinder at all times unless a regulator is in place.
- Cylinders must be stored in areas where they will not become overheated. Avoid storage near radiators, areas in direct sunlight, steam pipes and heat releasing equipment such as sterilizers.
- Do not store compressed gas cylinders in cold rooms or other areas with recirculating ventilation.
- Cylinders must be segregated as described below in the “Secondary Storage Considerations: Chemical Segregation" section
- Cylinders must be transported as described below in the “Chemical Transport” section
Toxic, flammable, and oxidizing gases have additional storage requirements and limits:
- Cylinders of toxic and reactive gases must be stored and used in a fume hood or ventilated gas cabinet designed for this purpose. See the Hazardous and Highly Toxic Gases SOP for additional requirements for the storage and use of these gases. Certain gases may not be purchased and used on campus without EHRS review and approval.
The Hazard Control Plan for your lab's procedures involving pyrophoric compounds will detail any specific storage conditions and restrictions that apply to your materials.
Proper storage and transport of pyrophoric chemicals must be determined by assessing all of the hazards and physical properties of the chemical.
See SOP: Pyrophoric Chemicals for more information.
The Hazard Control Plan for your lab's procedures involving explosive compounds will detail any specific storage conditions and restrictions that apply to your materials.
Proper storage and transport of explosive chemicals must be determined by assessing all of the hazards and physical properties of the chemical.
See SOP: Explosive Compounds for more information.
Secondary Storage Considerations: Chemical Segregation
Within each primary storage location (shelf, cabinet, etc.) incompatible materials may not be stored together without appropriate segregation.
Do not segregate chemical classes into separate rooms unless they will only be used in that room. Segregation that disrupts normal work flow or requires more frequent transport of chemicals between labs will increase the probability of a chemical spill.
Incompatible materials should be stored in separate cabinets whenever possible. For example: Acids and bases would be kept in separate corrosive liquids storage cabinets. However, when that is not possible, secondary containment bins can be used to segregate the incompatible materials. The secondary containment must be large enough to accommodate the volume of the largest container stored within. Once separated into hazard classes, chemicals may be stored alphabetically or by other systems such as by carbon number.
See this Tip Sheet for guidance on using ChemTracker to filter your chemical inventory by storage group.
Segregate solid chemicals from liquid chemicals.
Keep the following classes of solid chemicals segregated from each other in separate cabinets or secondary containers.
- oxidizing solids
- flammable solids
- water reactive solids
- all others solids
Segregate liquid chemicals from solid chemicals.
Keep the following classes of liquid chemicals segregated from each other in separate cabinets or secondary containers.
- acid liquids
- alkaline liquids
- oxidizing liquids
- flammable or combustible liquids
- pyrophoric & water-reactive liquids
- all other liquids
See "Primary Storage Locations" (above) and "Transportation of Chemicals" (below) for more information about proper management of compressed gas cylinders.
The following compressed gas types must be stored separately from each other:
- toxic gases
- flammable gases
- oxidizing gases*
- empty cylinders must be stored separately from full or partially-full cylinders
*Oxidizing gas must be separated by a distance of at least 20 feet from fuel gas cylinders or a highly combustible material such as, but not limited to, oil, grease, flammable gas or a source of ignition, or be separated from the material by a noncombustible wall, not less than five feet high, having a fire resistance rating of one hour. All cylinders shall be stored away from heat in excess of 125° Fahrenheit. See above for more information about compressed gas cylinder storage.
Designated Areas are a concept that applies to labs that mostly use non-hazardous or very low-hazard materials but may have one or two higher hazard chemicals. In these labs, PPE requirements and other chemical hygiene practices may not be uniform throughout the lab space. In such labs, all locations within the laboratory where acutely toxic, carcinogenic, or reproductive hazards are handled must be demarcated with designated area caution tape. Preprinted tape is available from EHRS. Alternately the lab worker may write “designated area” on yellow tape. Areas that must be designated include all fume hoods, sinks, and bench tops where the acutely toxic, carcinogenic, or reproductive hazards are handled. The tape should be used in the same manner as radiation caution tape; the lab worker may designate an area only during the time the chemical is used and then remove it or may permanently designate an area and leave the tape in place.
For labs where hazardous chemicals are routinely used throughout the room, the entire lab space may be deemed a “Designated Area”. This is accomplished by including a “Designated Area” sticker on the lab room sign and by enforcing uniform practices for chemical-handling, PPE, housekeeping, and decontamination throughout the entire lab space.
Chemical Stability
Stability refers to the susceptibility of the chemical to undergo dangerous decomposition.
For the purposes of this section of the CHP, we are referring to the likelihood that chemicals will undergo decomposition or react (spontaneously or gradually) during normal storage in the lab.
The manner in which a chemical is stored and the length of time that it may remain stored in the laboratory is determined by the stability of the material.
Some chemicals are inherently unstable, while others may become more reactive in the presence of heat, air, water, light or other contaminants. For this reason, it is important to understand which chemicals stored in your lab are, or may become, unstable and under what conditions this may occur.
Refer to the safety data sheet or ask your supervisor or EHRS for help determining whether a chemical may be unstable.
All potentially unstable chemicals must be dated upon receipt and upon opening, and they must be discarded through EHRS immediately whenever any of these conditions exist:
- The chemical is no longer needed for current research needs
- The expiration date specified by the manufacturer on the chemical label has been reached
- The storage duration specified for this chemical in Penn’s Chemical Hygiene plan has been reached. (Varies depending on hazard , see below)
- The purity of the material becomes suspect due to known contamination or changes in the physical appearance or activity of the chemical are observed
- The chemical container or cap is damaged
- The chemical is discovered in the lab with no “received” or “opened” date on the label
For specific storage limitations and expiration intervals for peroxide-formers and explosive compounds see the following SOPs and sections in Penn’s Chemical Hygiene Plan:
Peroxide-forming chemicals are a class of materials that have the ability to form shock-sensitive and explosive peroxide crystals. When triggered by friction or shock the peroxides will explode.
Peroxides form after exposure to air. The rate of peroxide formation is dependent on the specific chemical, the amount of air exposure and whether the chemical contains and inhibitor to retard peroxide formation.
See Peroxide-Forming Chemicals SOP for categories of peroxide-formers and their storage limitations.
Examples:
- Peroxide-formers (including THF, diethyl ether, potassium metal, and others)
- Auto-polymerizing (vigorously condensing) compounds (including vinyl chloride, butadiene, and others)
Definition from Prudent Practices in the Laboratory: Handling and Management of Chemical Hazards (section 4.D.3.1 Explosive Hazards) The National Academies Press: Washington, DC, 2011.
“An explosive is any chemical compound or mechanical mixture that, when subjected to heat, impact, friction, detonation, or other suitable initiation, undergoes rapid chemical change, evolving large volumes of highly heated gases—typically nitrogen or CO2—that exert pressure on the surrounding medium. The term applies to materials that either detonate or deflagrate.”
See Explosive (and Potentially Explosive) Compounds SOP for more information.
Storage limit guidance for explosive compounds (from SOP):
- Write on the container label the date that the material was received and the date the container was opened.
- Unless an inhibitor was added by the manufacturer, unopened containers of potentially explosive and shock-sensitive materials must be discarded after 1 year. Open containers of potentially explosive and shock-sensitive materials must be discarded within 6 months of opening.
- Store and work with explosive compounds in areas designated especially for that work. Remove all other chemicals and hazardous materials from the work area.
- Explosive classes of compounds synthesized in the lab have other storage requirements. See the SOP for details.
Examples of explosive and potentially explosive classes of chemicals:
- Shock-sensitive materials
- Air, water, and light sensitive materials
- Organic peroxides
- Azides
- Nitrates
- Explosive salts
- Perchlorates
- Fulminates
- Picrates
- And others (see SOP)
Transportation of Chemicals
The transportation of hazardous chemicals in laboratory buildings provides the greatest potential for chemical exposure to the building occupants. Spills occurring outside storerooms and laboratories may lead to hazardous concentrations of vapors and gases being distributed throughout the building. Always use a carrier when transporting reagent containers by hand.
General Chemical Transport Guidelines:
- Chemicals, substances and research materials must be clearly labeled with the correct chemical name when transported. Hand-written labels are acceptable; chemical formulas and structural formulas are not acceptable (except for small quantities of compounds synthesized in the laboratory).
- Persons transporting chemicals outside of the laboratory must wear safety glasses and one disposable glove for handling the chemical container and carrier. One hand must remain ungloved for operating door handles and elevator buttons.
- An appropriate chemical cart must be used whenever chemicals cannot easily be transported in a carrier using one hand. The cart must have sides, on each shelf, that are high enough to retain the containers. Cart wheels must be large enough to prevent the carts from being caught in floor cracks and door and elevator thresholds.
- If two gloves are worn while operating a chemical cart, one glove must be removed before operating door handles and elevator buttons or a second person who is not wearing gloves must accompany the person operating the cart.
- Freight elevators shall be used where available to transport hazardous materials. Under no circumstances are passenger elevators to be used for the transportation of hazardous materials if freight elevators are available.
Flammable Liquids Transport
- Original containers of flammable liquids shall be placed in a secondary container or acid-carrying bucket.
- No more than 5 gallons of flammable liquids in glass containers shall be transported on the freight elevator unless the original shipping carton (box) is used and the materials are on an appropriate cart.
More information about the safe use of flammable liquids is available in the Flammable Liquids SOP.
Corrosives or Oxidizing Materials Transport
- Glass containers liquid acids and bases must be placed in a secondary container or acid-carrying bucket.
- Incompatible chemicals, for example chromic acid (oxidizing acid) and ethyl acetate (organic solvent), may not be transported on the same cart unless they are in original shipping cartons and physically separated.
More information about safe use of corrosive liquids is available in the Corrosive Chemicals SOP.
More information about safe use of oxidizing chemicals is available in the Strong Oxidizers SOP.
Water-Reactive, Pyrophoric, and Acutely Toxic Substances Transport
- Wherever possible, use the original outside shipping containers (packaging) when transporting water-reactive, pyrophoric, or acutely toxic chemicals.
- Once opened, water-reactive, pyrophoric, and acutely toxic chemicals must be placed in a rigid secondary container or acid carrying bucket for transporting.
More information about safe use of these materials is available in the following SOPs: Water-Reactive Chemicals SOP, Pyrophoric Chemicals SOP, Acutely Toxic Chemicals SOP
Compressed Gases Transport
- Transport compressed gas cylinders using only equipment designed for this function. Regular hand trucks may not be used for cylinder transport.
- Never lift, carry, or "walk" cylinders by hand.
- The protective valve cap must be in place during transport.
- Never transport a cylinder with a regulator in place.
More information about the safe use of compressed gases is available in the Compressed Gases SOP and the Hazardous and Highly Toxic Gases SOP.
Contact EHRS for assistance if you need to move a large amount of hazardous material outside of the building (for example: for a lab relocation).
When hazardous materials must be transported outside of the building by lab personnel, all of the transportation requirements described above for each hazard type shall apply.
Additional precautions must be taken to avoid cart-tipping, container collision, and container upset, which are more likely to occur when moving chemicals down sidewalks and across streets. Use secondary containment, and secure and segregate chemical containers appropriately to prevent spills. All containers must be clearly labeled with the contents, their hazards, and the name of the person responsible for their transport.
Lab workers may not move pressurized dewars of liquid cryogens outside of the building.
For information about transporting animals, biologicals, and chemicals on the TRL Shuttle see: /health-safety/shipping/trl-shuttle
Revised 11/2017
Each laboratory has a room sign that provides safety information to visitors, housekeeping and maintenance personnel (see Figure 1). Some biomedical laboratory buildings will have individual bench signs. The principal investigator is responsible for assuring that appropriate warning information is included on this sign.
Perelman School of Medicine lab should use this link for room signs to access a Word document to be sent to PSOM Space Planning and Operations.
All other labs should click here.
The Rooms sign is composed of four sections. Section one contains the room number and department name. Section two includes the names of the principal investigator and researchers. Section three provides warning information to visitors, housekeeping and maintenance personnel. Special labels are available that are placed into the boxes of this section. The labels contain icons alerting the reader that one of the following conditions exists inside the laboratory:
- CAUTION-BIOHAZARD
- CAUTION-DESIGNATED AREA WITHIN
- CAUTION-RADIOACTIVE MATERIALS
- CAUTION-LASER
- CAUTION-MAGNETIC FIELD
- CAUTION-DESIGNATED AREA LABORATORY
Section four provides safety department phone numbers.

Students, faculty, staff and administrators shall not enter a designated area of a lab unless they are familiar with the facility or accompanied by an authorized user of the facility.
Custodians are permitted to enter restricted or designated areas to perform routine tasks. However, labeled waste containers, other research equipment or materials, must not be handled.
Other support services, such as: University Police, Physical Plant, Safety Personnel, etc., are permitted to enter a designated areas. Support services should avoid disturbing the following areas:
- fume hoods
- biological safety cabinets
- sinks
- placarded equipment
- chemicals or materials on laboratory benches
Support personnel shall contact an authorized user of the facility or EHRS before performing work that may involve any of the above items. Contact EHRS if emergency response or service is required in a designated area or in a designated area laboratory.
Immediately report any unusual conditions to EHRS or University Police, such as:
- spills
- leaks
- fires
- injury
- contamination
See Chemical Waste Guidelines for information on handling chemical lab waste and other hazardous chemical waste streams.
See the Biosafety Manual for information on infectious waste streams.
Revised 11/2017
The safety data sheet (SDS) is a hazard communication tool that provides details on important aspects of chemical use, handling, and storage. Review both the appropriate Standard Operating Procedure and the SDS when working with a chemical for the first time or when training staff.
The Office of Environmental Health and Radiation Safety (EHRS) maintains Safety Data Sheets (SDSs) for all chemicals used at the University. SDSs can be obtained by calling EHRS at 215-898-4453, emailing EHRS or ordered through the EHRS website.
The OSHA Hazard Communication standard (29 CFR 1910.1200) requires manufacturers to provide SDSs at no cost. Information is divided into the sixteen sections described below.
Information in a standardized GHS-compliant SDS is presented using the following 16 headings in the order given below:
| Section | Title | Description |
| 1 | Identification | Product identifier used on the label and any other common names or synonyms by which the substance is known and the name, address, phone number of the manufacturer, importer, or other responsible party, and emergency phone number. |
| 2 | Hazard Identification | The hazards of the chemical and the appropriate warning information associated with those hazards. All labeling information. |
| 3 | Composition/ Ingredients | Chemical and common name, CAS number, impurities, and concentration of ingredients in a mixture. |
| 4 | First Aid Measures | Describes the initial care that should be given by untrained responders to an individual who has been exposed to the chemical. |
| 5 | Fire Fighting Measures | Recommendations for fighting a fire caused by the chemical. |
| 6 | Accidental Release Measures | Recommendations on the appropriate response to spills, leaks, or releases, including containment and cleanup practices to prevent or minimize exposure to people, properties, or the environment. |
| 7 | Handling and Storage | Guidance on the safe handling practices and conditions for safe storage of chemicals. |
| 8 | Exposure Controls/Personal Protection | Indicates the exposure limits, engineering controls, and personal protective measures that can be used to minimize worker exposure. |
| 9 | Physical and Chemical Properties | Identifies physical and chemical properties associated with the substance or mixture. |
| 10 | Stability and Reactivity | Describes the reactivity hazards of the chemical and the chemical stability information. This section is broken into three parts: reactivity, chemical stability, and other. |
| 11 | Toxicological Information | Identifies toxicological and health effects information or indicates that such data are not available. |
| 12 | Ecological Information | (Non-mandatory) Provides information to evaluate the environmental impact of the chemical(s) if it were released to the environment. |
| 13 | Disposal Considerations | (Non-mandatory) Provides guidance on proper disposal practices, recycling or reclamation of the chemical(s) or its container, and safe handling practices. |
| 14 | Transport Information | (Non-mandatory) Provides guidance on classification information for shipping and transporting of hazardous chemical(s) by road, air, rail, or sea. |
| 15 | Regulatory Information | (Non-mandatory) Identifies the safety, health, and environmental regulations specific for the product that is not indicated anywhere else on the SDS. |
| 16 | Other Information | This section indicates when the SDS was prepared or when the last known revision was made. |
Safety Data Sheets are only one source of chemical safety information. In a research setting, more information about the chemical may be needed to conduct a thorough hazard assessment for the process involving the chemical in question. If you are trying to determine chemical reactivity and incompatibility hazards or are planning hazard controls for a procedure, work with your PI and EHRS in addition to consulting a safety data sheet.
These are a few additional resources for chemical safety information:
Pub Chem Laboratory Chemical Safety Summaries
http://hdl.library.upenn.edu/1017/13802
Sittig’s Handbook of Toxic and Hazardous Chemicals
https://app.knovel.com/web/toc.v/cid:kpSHTHCC12/viewerType:toc/root_slug:sittigs-handbook-of
Hazardous Substances Data Bank (HSDB)
http://toxnet.nlm.nih.gov/newtoxnet/hsdb.htm
NIOSH International Chemical Safety Cards
http://www.cdc.gov/niosh/ipcsneng/nengname.html
CAMEO database of Hazardous Materials (helpful with chemical reactivity)
Revised 5/2019
Do not hesitate to call EHRS for assistance with spill cleanup:
215-898-4453 24-hours/day phone response
See the Chemical Spill section on the Emergency Information page for more information.
All spills of highly hazardous materials or spills that result in (or could have resulted in) personal exposure to hazardous materials will require the lab personnel to complete a Lab Safety Event Data Collection Form. After you notify EHRS of the spill, an EHRS staff member will instruct you to complete the form. The intention of this form is to collect complete information as soon as possible after an event. EHRS uses this information to understand how we can prevent recurrence or prevent similar events from occurring in other labs.
Most chemical spills can be avoided through good lab housekeeping practices such as:
- Storing and closing containers properly
- Minimizing clutter on the bench
- Using chemical-transport buckets when carrying chemicals outside the lab
- Using secondary containment in spill-prone areas or for high-volume or high-hazard chemicals
- Avoiding distractions while working
Some spills can be safely handled by lab workers if they have the proper cleanup materials, PPE, training, and the hazards of the spilled substance are well understood. If you have any doubts or concerns about cleaning up a spill in the lab, contact EHRS for guidance or assistance.
General spill procedures
- Use a dustpan and brush for broken glass and/or solid spills. Do not pick up broken glass by hand.
- Wipe up solids carefully to avoid inhaling powders.
- At a minimum, standard lab attire and PPE are required when cleaning up spills. Some spills may require additional PPE such as chemical splash goggles or heavy duty chemical-resistant gloves.
- Decontaminate area with soap and water after clean-up. Place residue in a container for waste collection.
- Contact EHRS for disposal information.
- For specific spill cleanup information, contact your supervisor, instructor, or EHRS.
Absorbents and neutralizers
Most small spills do not need to be neutralized before cleanup.
The absorbent and neutralizers needed for the cleanup will depend on the hazards and properties of the material spilled. Consult the SDS for any new chemical you are working with. Anticipate spills by having the appropriate spill materials on hand.
Never use cellulose-based absorbents such as paper towels to absorb strong oxidizers (like nitric acid or sulfuric acid). Violent chemical reactions and/or fire may result.
Use an absorbent material that will neutralize the spill if available. Examples of spill cleanup materials include:
- paper towels
- spill pads
- sodium bicarbonate for acids
- powdered citric acid for bases
- clay-type absorbents such as "Oil-Dri","Zorb-All" or "Speedi-Dri" for acid chlorides
Revised 11/2017
See the Emergencies Involving Injuries section of this website for locations and contact information for medical care
All lab events result in injury or exposure (or near misses that could have resulted in injury or exposure) will require the lab personnel to complete a Lab Safety Event Data Collection Form. After you notify EHRS of the event, an EHRS staff member will instruct you to complete the form. The intention of this form is to collect complete information as soon as possible after an event. EHRS uses this information to understand how we can prevent recurrence or prevent similar events from occurring in other labs.
Medical Response for Life-Threatening Injuries or Chemical Exposures
Do not move a seriously injured person unless they are in further danger.
DIAL 511 from a Penn Campus Phone
Or 215-573-3333 from a Cell Phone
Tell the dispatcher the location and the nature of the emergency.
Notify the injured person’s direct supervisor or instructor and EHRS as soon as possible after immediate medical needs have been addressed.
Employees and students must notify their immediate supervisor or instructor of all illness and injuries related to exposure to hazardous chemicals. Contact your supervisor, instructor, or EHRS if you have any questions regarding the procedure for treating a non-serious injury or illness.
Treatment or consultation for non-life-threatening injury, illness, or exposures.
See the Emergencies Involving Injuries section of this website for locations and contact information for medical care.
Revised 11/2017
All lab events result in injury or exposure (or near misses that could have resulted in injury or exposure) will require the lab personnel to complete a Lab Safety Event Data Collection Form. After you notify EHRS of the event, an EHRS staff member will instruct you to complete the form. The intention of this form is to collect complete information as soon as possible after an event. EHRS uses this information to understand how we can prevent recurrence or prevent similar events from occurring in other labs.
- Do what is necessary to protect life.
- If assisting a contaminated person, use appropriate PPE to avoid contaminating yourself.
- Quickly remove all contaminated clothing while using the safety shower or other available source of water. Immediately flood the affected body area in tepid or cold water for at least 15 minutes. If there is no visible burn, scrub area with warm water and soap. Remove all jewelry to facilitate removal of any residual material. Wash off chemical with water but do not use neutralizing chemicals, unguents, creams, lotions, or salves unless a CHP Fact Sheet or Hazard Control Plan specifically advises it (e.g. Calcium gluconate for HF exposures)
- Do not move an injured person unless they are in further danger.
- If the person is seriously injured or unable to move on their own, call Penn Police to request an ambulance. 511 (University of Pennsylvania phone only), 215.573.3333 (any phone)
- Identify all chemicals involved in the exposure
- Contact the injured person’s supervisor or instructor as soon as possible
- Contact EHRS as soon as possible after immediate medical needs have been addressed or if there are questions about where to go for medical care
- All chemical exposures must be treated by a health care provider. See the Emergencies Involving Injuries section of this websitefor information on where to go for medical care and consultation.
All exposures to hydrofluoric acid (HF) acid must be treated immediately.
Hydrofluoric acid exposures require special treatment. An HF spill kit must be available in all labs where HF is handled. See the Hydrofluoric Acid Fact Sheet for more information.
Irrigate eyes using the emergency eye wash. Hold eyes open with clean hands and flush continuously with cool water for at least 15 minutes. Simultaneously, check for and remove contact lenses.
All chemical exposures must be treated by a health care provider. See the Emergencies Involving Injuries section of this website for information on where to go for medical care and consultation.
- Extinguish burning clothing by dousing with cold water or use the emergency shower or the drop-and-roll technique.
- Remove contaminated clothing. If possible, send clothing with the victim. Wrap injured person to prevent shock.
- Anyone overcome with smoke or chemical fumes should be removed to uncontaminated air and treated for shock.
If certified, follow standard CPR protocols. Get medical attention promptly. See the Emergencies Involving Injuries section of this website for information on where to go for medical care and consultation.
Revised 11/2017
All lab events result in fire (or near misses that could have resulted in fire) will require the lab personnel to complete a Lab Safety Event Data Collection Form. After you notify EHRS of the event, an EHRS staff member will instruct you to complete the form. The intention of this form is to collect complete information as soon as possible after an event. EHRS uses this information to understand how we can prevent recurrence or prevent similar events from occurring in other labs.
During a fire emergency, the University of Pennsylvania’s Division of Public Safety – Fire and Emergency Services (FES) emphasizes safe evacuation as top priority. If you discover a fire or fire-related emergency, such as abnormal heating of material, hazardous gas leaks, hazardous material or flammable liquid spill, smoke, or odor of burning; immediately follow the procedures on the Emergency Info: Fire page.
Incipient fires with a mundane fuel source (e.g. pure flammable solvents, nonhazardous lab trash) may be fought to assist oneself or another to evacuate, or control a small fire only if:
- You have received hands-on training at Penn on how to use a portable fire extinguisher
- It is safe to do so and the fire is not located between you and your exit.
- The fire is still contained to the original fuel source and has not begun to spread.
- You are not alone.
- The appropriate type of extinguisher is available.
Small incipient fires with an exotic fuel source (e.g. pyrophoric or explosive chemicals, reaction mixtures containing highly corrosive, toxic, or other hazardous chemicals) may be attempted to be immediately smothered using sand, powdered lime, or a similar dry extinguishing agent. However, if this does not immediately put out the fire, do not continue fighting the fire; evacuate the area and notify emergency services as outlined on the Emergency Info: Fire page.
If the fire alarms are ringing in your building:
- Evacuate the building.
- Move away from the building to a designated area.
- Stay clear of driveways, sidewalks and other means of access to the building.
If you are a supervisor, account for your employees and report any missing persons to the emergency personnel at the scene. Assist emergency personnel as may be requested.
Do not reenter the building until directed to do so. Follow any special procedures established for your unit.
- Extinguish burning clothing by dousing with cold water or use the emergency shower or the drop-and-roll technique.
- Remove contaminated clothing. If possible, send clothing with the victim. Wrap injured person to prevent shock.
- Anyone overcome with smoke or chemical fumes should be removed to uncontaminated air and treated for shock.
If certified, follow standard CPR protocols. Get medical attention promptly. See the Emergencies Involving Injuries section of this website for information on where to go for medical care and consultation.
Revised 11/2017
Revised 11/2017
The University is required to advise you of your rights regarding the Hazard Communication Standard, Personal Protective Equipment Standard and Occupational Exposure to Hazardous Chemicals in the Laboratory. This manual meets these requirements in part. In addition, a standard OSHA "Notice to Employee" poster will be posted at locations where notices are normally posted. It is to your advantage to know your rights. Take time to read the "Notice to Employee" form posted in your work area.
Employees who may be exposed to hazardous chemicals have access to the following information where appropriate:
- Chemical exposure information
- Workplace chemical lists
- Safety data sheets
In addition, employees and students shall receive training on the hazards of chemicals and on the measures they can take to protect themselves from those hazards.
You have the right to file a complaint against the University regarding alleged violations of the Hazard Communication Standard or Chemical Hygiene Plan. If you file a complaint, the Act protects you from:
- Discharge
- Cause for discharge
- Discipline
- Discrimination
- Loss of pay, position, seniority or benefits
Alleged violations of any OSHA standard should be referred to your supervisor, instructor, or the Office of Environmental Health and Radiation Safety. However, you always have the right to file a complaint with the Occupational Safety and Health Administration (OSHA).
The employer must assess the workplace to determine if hazards are present, or are likely to be present, which necessitate the use of personal protective equipment. The Principal Investigator fulfills this responsibility by completing a Chemical Hygiene Work Plan (CWHP) as described in Section II of this document. A copy of your lab's CWHP is available in your lab's BioRAFT profile.
The Principal Investigator is responsible to select and provide employees with routine personal protective equipment appropriate for laboratory work (e.g. lab coats, disposable gloves, safety glasses, face shields and other similar items along with laundry facilities or service for lab coats). Students may be required to purchase common items such as laboratory aprons or goggles. The Principal Investigator in consultation with the Office of Environmental Health and Radiation Safety will select non routine personal protective equipment such as respirators, chemical protective gloves, and chemical protective clothing.
Principal Investigators shall assure that training in the use of routine laboratory personal protective equipment is provided. This training is provided through attendance at the program "Introduction to Laboratory Safety at Penn", provided by the Office of Environmental Health and Radiation Safety and through laboratory specific training provided by the Principal Investigator.
The Office of Environmental Health and Radiation Safety in conjunction with the Principal Investigator will provide training in the use of non-routine personnel protective equipment.
Upon appointment, the University will provide education and training programs for employees using or handling chemicals. Additional instruction is required whenever the potential for exposure to hazardous chemicals is altered or whenever new information concerning a chemical is received. New or newly assigned employees must be provided training before working with, or in a work area containing hazardous chemicals. For students, training may be required for each course. Training programs shall include, as appropriate, the following:
- Interpreting labels and SDSs
- Location of hazardous chemicals
- A description of the acute and chronic effects of chemicals
- Safe handling procedures
- Personal protective equipment
- Cleanup procedures
- Waste disposal
- Emergency Procedures
In an area or laboratory where a large variety of hazardous chemicals are stored or used, the University may substitute generic training for chemical specific training. The contents of this manual meet the requirements of 29 CFR 1910.1200, Hazard Communication Standard and 29 CFR 1910.1450, the Lab Standard.
The University is required to keep a record of training sessions provided to employees. EHRS Training is documented through Knowledge Link. The Principal Investigator is responsible for documenting hazard-specific safety training provided in the laboratory.
If you do not understand the material provided or discussed, contact your supervisor, instructor or EHRS.
Revised 8/2023
Standard Operating Procedures (SOPs), Fact Sheets, and Hazard Control Plans
Standard operating procedures (SOP) and Fact Sheets are intended to provide you with general guidance on how to safely work with a specific class of chemicals or type of hazard.
Standard Operating Procedures give the requirements for working with a general hazard class (such as "Acutely Toxic Chemicals")
Fact Sheets give guidance on a specific substance (such as "Elemental Mercury").
While SOPs & Fact Sheets provide only general guidance, observance of all the safety practices listed in them is mandatory. If compliance with all the requirements of a specific standard operating procedure is not possible, then the principal investigator must develop a written procedure that will be used in its place. This alternate procedure must provide the same level of protection as the SOP it replaces. (See "Hazard Control Plans" below.)
The Office of Environmental Health and Radiation Safety can provide guidance for the development of alternate procedures.
All lab members who work in a lab where hazardous materials are stored or used must read the SOPs and Fact Sheets that apply to the hazards in their lab. For more information about this, see Section I: Introduction to the Chemical Hygiene Plan (+"What is the Chemical Hygiene Work Plan?")
The requirements detailed in the SOPs and Fact Sheets are mandatory for working with hazardous materials in any quantity large enough to pose a risk to the worker. For some tasks, additional controls may also be required. EHRS can assist with risk assessment and with developing additional controls on request.
Questions concerning proper handling procedures should be directed to your Principal Investigator or to Environmental Health and Radiation Safety.
Click on the links below to access the Chemical Hygiene Plan SOPs and Fact Sheets
Hazard Control Plans (HCPs)
The SOPs and Fact Sheets in the Chemical Hygiene Plan provide guidance on broad categories of hazards. A Hazard Control Plan (HCP), however, is a lab-specific and/or task-specific SOP that describes the hazards and controls for a particular procedure you will perform in your lab.
Anyone can complete the HCP, but the completed plan must be reviewed by the supervising PI.
An HCP should be written:
- For all hazardous (flammable, toxic, oxidizing, corrosive) compressed gases, explosives compounds, and/or pyrophoric materials
- Whenever a new hazard type is introduced in your work
- When the hazards of a procedure are unknown
- When the hazards of a task are considered particularly high.
- Before the hazardous task is performed for the first time.
Below are some examples of high-hazard situations involving chemical use. (From 2020 ACS Style Guide Chapter on Safety)
- Elevated pressure or temperature where apparatus or conditions could reasonably lead to a fire, explosion or loss of containment
- Oxygen at greater than 25% or oxygen/fuel mixtures which are ignitable
- Compounds with C:N ratio less than 5 carbons per nitrogen
- Oxidations or organic molecules, particularly at elevated temperature and/or gram scale or greater
- Processes with high exothermicity that could lead to run-away reaction
- Processes in which the energetics of scalability are insufficiently defined or require special cooling
- The addition of complexity (e.g. biological pathogens, nanoparticles, etc.) into the research.
Email your completed HCP to EHRS (ehrslaba@ehrs.upenn.edu). EHRS will upload the document to the documents section of your lab's BioRAFT profile.
Appendix A: References
References
- Altman, P. L., Dittmer, D. S., Biology Data Book, Vol. I-III, 2nd ed., Fed. of Am., Societies for Experimental Biology: Bethesda, MD., 1972
- Anon, Toxic and Hazardous Industrial Chemicals Safety Manual, international Technical Information Institute: Tokyo, Japan, 1979
- Bretherick, L. Handbook of Reactive Chemical Hazards, 2nd ed., Butterworths: Boston, MA., 1979
- Brodsky, A., editor, CRC Handbook of Radiation Measurement and Protection, CRC Press Inc: West Palm Beach, FL., 1978
- Carcinogens, U. S. Department of Health and Human Services, Public Health Service, National Toxicology Program, U. S. Government Printing Office, Washington, D. C., latest edition.
- Casarett, A., Radiation Biology, Prentice-Hall, Inc.,: Englewood Cliffs, NJ., 1968
- Casarett, L.J., Doull, J., Eds., Toxicology, MacMillan: New York, 1975
- Cember, H., Introduction to Health Physics, Pergamon Press: New York, 1969
- Deichmann, W. B., Gerarde, H.W., Toxicology of Drugs and Chemicals, 4th ed., Academic Press: New York, 1969
- Delaware Code, Title 16, Chapter 24, "Hazardous Chemical Information Act", State of Delaware: Dover, Delaware
- Documentation of the Threshold LImit Values for Substances in the Workroom Air and Supplemental Documentation, American Conference of Governmental Industrial Hygienist: Cincinnati, OH., (latest edition)
- Fire Protection Guide on Hazardous Materials, 7th ed., National Fire Protection Association: Boston, MA
- Goodman, L.S., Gilman, A., The Pharmacological Basis of Therapeutics, Macmillan: NY, 1975
- Gosselin, R.E., et al. Clinical Toxicology of Commercial Products: Acute Poisoning, 4th ed., Williams and Wilkins: Baltimore, MD, 1976
- Green, M.E., Turk, A., Safety in Working with Chemicals, Mcmilllan: New York, 1978
- The Hazard Communication Standard - A Guide Book, National Safety Council: Chicago, IL., 60611
- Hilado, C.J., Clark, S.W., Autoignition Temperatures of Organic Solvents, Chem. Eng.: NY, 1972, (19), 75-80
- Industrial Environmental-Its Evaluation and Control,U. S. Department of Health, Education and Welfare, Public Health Services, NIOSH, U.S. Printing Office: Washington, DC., Stock Number 017-001-00396-4, 1973.
- Industrial Ventilation, American Conference of Governmental Industrial Hygienist, Committee on Industrial Ventilation: Lansing, MI., (latest edition)
- Knoll, G., Radiation Detection and Measurement, John Wiley & Sons, NY, 1979
- Lewis, R.J., Ed. Registry of Toxic Effects of Chemical Substances, DHEW, (NIOSH), Publ. Microfiche, issued quarterly
- Lomis, T.A., Essentials of Toxicology, 3rd ed., Lea Febiger: Philadelphia, 1978
- Martin, A., Harbison, S., Radiation Protection, Chapman and Hall, Ltd.: London, England, 1979
- Moe, Lasuk, Schumacher, and Hunt, Radiation Safety Technician Training Course, Argonne National Laboratory, ANL-7291 Rev.1, Health and Safety, May,1972, available from NTIS, USDC; Springfield, VA., 22151
- Morgan, K., Turner, J., Principles of Radiation Protection, Robert E. Krieger Publishing Co.,: Huntington, NY., 1973
- Murr, G.D., ED.,, 2ND., Hazard in the Chemical Laboratory, 2nd ed., Chemical Society: London, 1972
- NIOSH OSHA Product Guide to Chemical Hazards, DHEW (NIOSH): September 1978, Publ. No. 78-210
- Olishifski, Julian B., McElroy, Frank E., Fundamentals of Industrial Hygiene, National Safety Council: Chicago, IL., 1976
- OSHA Safety and Health Standards (29CFR1910) - United States Department of Labor, OSHA, Government Printing Office: Washington, DC., (latest edition)
- PATTY, F. A., Industrial Hygiene and Toxicology: Volume II- Toxicology, Interscience Wiley: New York, 1980
- Proctor, N., Hughes, J., Chemical Hazards in the Workplace, Lippincott: Philadelphia, 1978
- Prudent Practices for Handling Hazardous Chemicals in Laboratories, National Research Council, National Academy Press: Washington, D.C., 1981
- Radiological Health Handbook, U.S.H.E.W., Public Health Service, F.D.A., Bureau of Radiological Health: Rockville, MD., 20852, available from USGPO Stock number 017-011-00043-0
- Safety in Academic Chemistry Laboratories- 3rd ed., Committee on Chemical Safety, American Chemical Society: Washington, D.C., 1979
- Sax, N.I., Dangerous Properties of Industrial Materials, 5th ed., Van Nostrand-Reinhold: New York, 1989
- Shapiro, J., Radiation Protection, Howard University Press: Cambridge, MA., 1981
- Sittig, M., Hazardous and Toxic Effects of Industrial Chemicals, Noyes Data Corporation: Park Ridge, NJ., 1979
- Furr, A.K., Ed, CRC Handbook of Laboratory, 3rd ed., CRC Press: Boca Raton, FL., 1990
- TLVs: Threshold Limit Values for Chemical Substances and Physical Agents in the Workroom Environmental and Intended Changes, TLV Airborne Contaminants Committee, American Conference of Governmental Industrial Hygienists: Cincinnati, OH., (latest edition)
- Walters, C.C., Ed., Safe Handling of Chemical Carcinogens, Mutagens, Teratogens, and Highly Toxic Substances, Ann Arbor Science Publishers, Inc., : Ann Arbor, MI., 1980, Vol. l
- Winholz, M., Ed., The Merch Index, 9th ed., Merck and Company: Rahway, NJ., 1976
- Zabetakis, M. G., Flammable Characteristics of Combustible Gases and Vapors, US Bureau of Mines Bulletin 627, 1965
Appendix B: The Chemical Hygiene Work Plan
The Chemical Hygiene Work Plan (CHWP) is now completed through Penn’s BioRAFT laboratory safety information system. In order to complete the CHWP, Lab Safety Coordinators or Principal Investigator (PI) must complete a short Lab Hazard Survey each year or when hazards in the lab change. The Lab Hazard Survey identifies relevant Standard Operating Procedures (SOPs) and Fact Sheets from Penn's Chemical Hygiene Plan to be included in the CHWP.
After the Lab Hazard Survey is completed, the PI will be prompted to certify the lab hazards. Upon PI approval, BioRAFT will prepare a Chemical Hygiene Work Plan (example shown below) with links to applicable SOPs and Fact Sheets for the lab. A link to the document will then be emailed by BioRAFT to all lab members.
If the lab has lab-specific Hazard Control Plans the PI should check the box indicating these exist and forward the lab-specific hazard control plans to EHRS. EHRS will attach the lab-specific hazard control plans to the documents tab of the lab's BioRAFT profile so that they available to all lab members.
A paper copy of the CHWP no longer needs to be posted in the lab.